


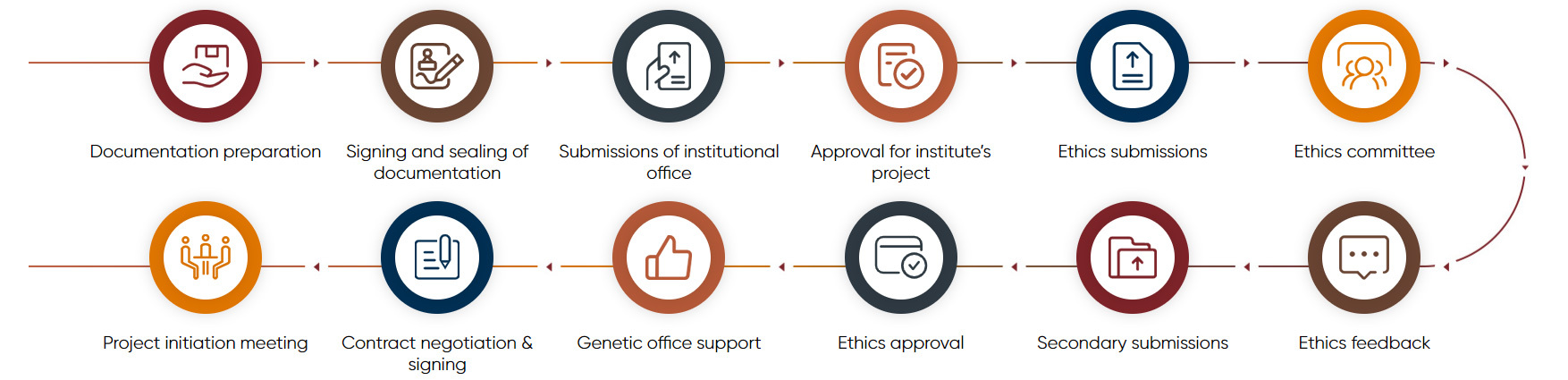














 Ethics submissions for project initiation and support for genetic office application
Ethics submissions for project initiation and support for genetic office application Subject screening and recruitment
Subject screening and recruitmentAccurately screen subjects according to protocol requirements
Huge investigator repository to efficiently expedite enrollment, especially in oncology research
Collaborate with relevant organizations and enterprises to provide multi-channel recruitment approaches, especially for rare diseases and chronic diseases
 Subject management
Subject managementTrack the whole process of patients’ treatment and follow-up according to ICH-GCP guidelines and clinical trial protocol requirements to effectively control the dropout rate
 Trial data entry & review
Trial data entry & reviewHelp investigators sort and complete the original records in an efficient and quality manner
Enter data and answer questions efficiently according to the trial and customer’s requirements to ensure the timely data entry
 Site management & maintenance
Site management & maintenanceInstitute and EC daily communication and maintenance
Study documentation management
Investigational drug and supplies management
Financial management of project
Support for monitoring,audit and inspection
 Reporting of AEs/SAEs
Reporting of AEs/SAEsAE/SAE progression tracking
Retention and filing of safety event documentation
Assist in AE/SAE information collection, recording and reporting

 Assist with query resolution
Assist with query resolution Site closure
Site closure On-site audit support
On-site audit support