


Acrostar SMO is a site management organization wholly-owned by the Novotech Health Holdings Group and set up branches and offices in Shanghai, Beijing, Nanjing, Guangzhou, Shenzhen, Hangzhou, etc.
Our business covers 28 provinces, autonomous regions and municipalities. Since its establishment in 2017, the business volume of Acrostar has been growing per year, making it one of the fastest growing SMO in China in recent years.
 Recruited 20,000+ subjects
Recruited 20,000+ subjects Cooperated with 1,000+ GCP departments nationwide
Cooperated with 1,000+ GCP departments nationwide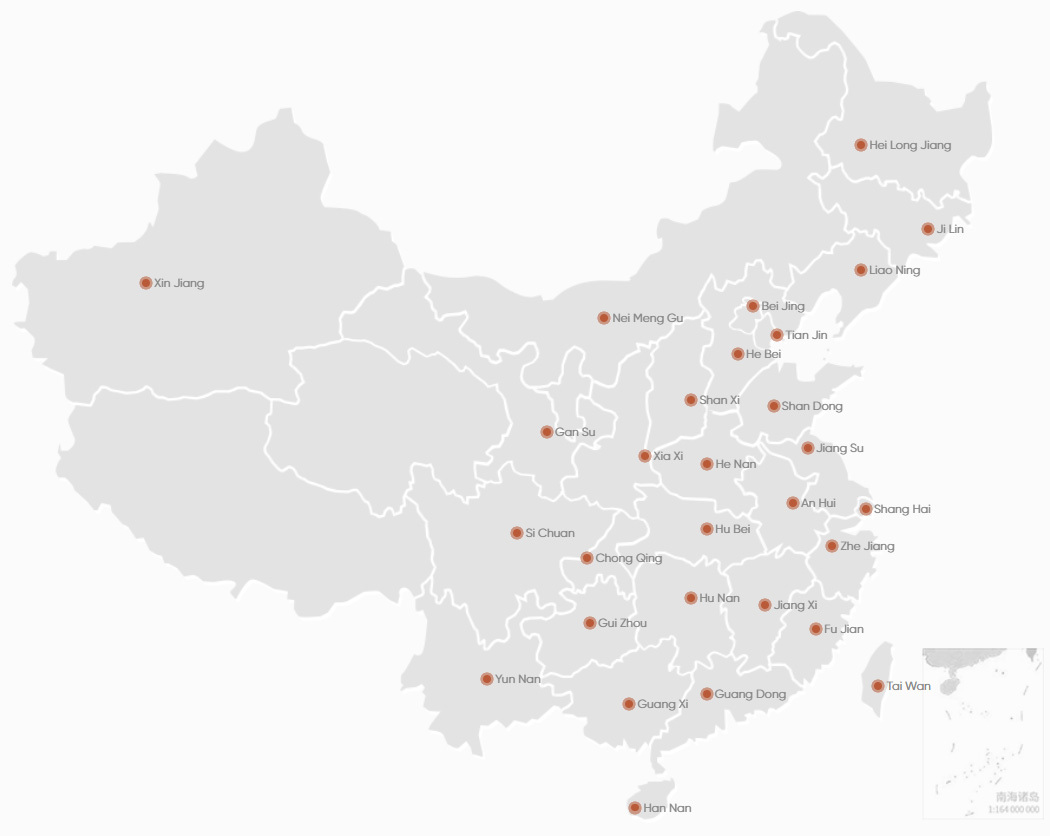
































 Tai Wan
Tai Wan Tai Wan Area×
Tai Wan Area× He Nan
He Nan He Nan Area×
He Nan Area× Hu Bei
Hu Bei Hu Bei Area×
Hu Bei Area× Xia Xi
Xia Xi Xia Xi Area×
Xia Xi Area× Chong Qing
Chong Qing Chong Qing Area×
Chong Qing Area× Si Chuan
Si Chuan Si Chuan Area×
Si Chuan Area× Gan Su
Gan Su Gan Su Area×
Gan Su Area× Xin Jiang
Xin Jiang Xin Jiang Area×
Xin Jiang Area× Han Nan
Han Nan Han Nan Area×
Han Nan Area× Guang Xi
Guang Xi Guang Xi Area×
Guang Xi Area× Hu Nan
Hu Nan Hu Nan Area×
Hu Nan Area× Yun Nan
Yun Nan Yun Nan Area×
Yun Nan Area× Fu Jian
Fu Jian Fu Jian Area×
Fu Jian Area× Jiang Xi
Jiang Xi Jiang Xi Area×
Jiang Xi Area× Guang Dong
Guang Dong Guang Dong Area×
Guang Dong Area× Gui Zhou
Gui Zhou Gui Zhou Area×
Gui Zhou Area× Zhe Jiang
Zhe Jiang Zhe Jiang Area×
Zhe Jiang Area× Shan Dong
Shan Dong Shan Dong Area×
Shan Dong Area× An Hui
An Hui An Hui Area×
An Hui Area× Shang Hai
Shang Hai Jiang Su
Jiang Su Jiang Su Area×
Jiang Su Area× Tian Jin
Tian Jin Tian Jin Area×
Tian Jin Area× Shan Xi
Shan Xi Shan Xi Area×
Shan Xi Area× He Bei
He Bei He Bei Area×
He Bei Area× Bei Jing
Bei Jing Bei Jing Area×
Bei Jing Area× Ji Lin
Ji Lin Ji Lin Area×
Ji Lin Area× Liao Ning
Liao Ning Liao Ning Area×
Liao Ning Area× Hei Long Jiang
Hei Long Jiang Hei Long Jiang Area×
Hei Long Jiang Area× Nei Meng Gu
Nei Meng Gu Nei Meng Gu Area×
Nei Meng Gu Area×- Passed on-site inspection by NMPA for the first time.
- Transformation of operations from Phase I to Phase II/III;
- Undertaking the first multinational drug company oncology trial;
- Undertaking the first cell therapy (CAR-T) project.
- The company was renamed Acrostar site management Co.,Ltd. Registered in Nanjing;
- More than 60 cooperative companies, including 30 foreign-funded enterprises;
- Became a preferred supplier of 12 GCP hospital.
- Expanded business scope to 27 provinces, autonomous regions and municipalities. And covering more than 60 cities, all with full-time CRCs;
- The company developed a talent training plan to strengthen the staff sense of belonging.
- Cooperation with over 400 GCP centres, and adding to the list of preferred supplier of dozens of hospitals;
- The number of collaborating clients exceeded 150.
- Overcoming the difficulties of a three-year epidemic. Multiple project teams prepare for NMPA verification;
- Improve the team structure, bring in excellent managers and strengthen the management team;
- Became a preferred supplier of several GCP centres.
- Supporting product approvals and market launches for multiple projects in development;
- Introduced and built project management system;
- Completed verification of several MNC companies and became their preferred supplier, with a 20% growth in business from the previous year.